Pharma & Chemicals
Pharmaceutical traceability at the heart of your compliance
We support pharmaceutical laboratories and chemical industries in digitizing and securing their traceability, to ensure regulatory compliance and optimize their production cycles.
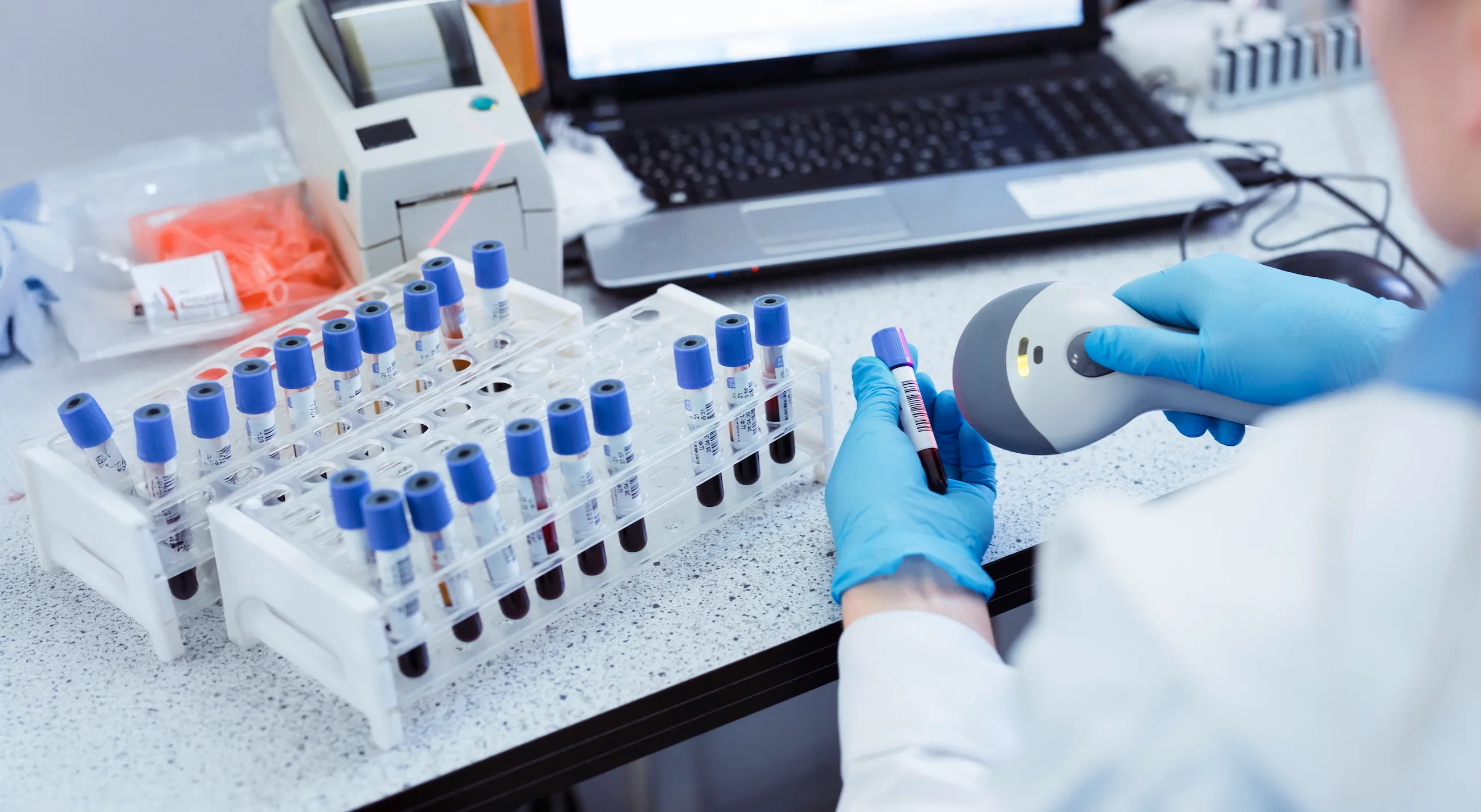
The ability to respond effectively to various challenges
Solid offers RFID, UHF, NFC, and BLE solutions to digitize your workflows and drastically reduce low-value-added tasks. The location and management of samples, equipment, and time are ongoing challenges for the pharmaceutical industry. Our solutions, thanks to the intelligent use of RFID technology combined with barcode/QR code technology, offer data centralization and digitalized management by automating scans and GMP report generation. Ensuring the lifecycle of your samples will no longer be a constraint. To enhance traceability in the pharmaceutical industry, our solutions integrate easily with your LIMS/MES systems thanks to our APIs.
Manual entry may expose you to discrepancies during audits.
The management of scans, reconciliations, and manual reminders mobilizes resources without any direct impact on R&D.
Test results and sample histories are often stored without encryption or detailed logging, exposing them to the risk of tampering.
Difficulty tracking raw materials, consumables, and samples throughout the production process.
Discover how our solutions adapt to your business reality
Clinical sample management
Clinical sample management
Instant reading of samples as they leave the refrigerators for fast transfer without breaking the cold chain, in complete safety: temperature alerts and regulatory compliance.
Internal and external audits
Internal and external audits
One-click extraction of usage histories, transfers, and validations, to respond quickly to the relevant authorities.
Equipment Traceability and Inventories
Equipment Traceability and Inventories
Accurately determine the location of your machines so you no longer waste time looking for them. Take inventory of your machines using RFID technology: 300 m² – 500 assets – 4 minutes.
Management of your products/bottles on shelves or in your cabinets
Management of your products/bottles on shelves or in your cabinets
Locate all your products and bottles on your shelves or in your cabinets with centimeter-level accuracy. Efficient restocking management and real-time inventory.

Our solutions for your industry

Our customers have asked us these questions
Can the inventory be taken without stopping work?
Yes. Thanks to RFID technology, you can carry out your inventory checks in real time without interrupting your teams. RFID chips enable contactless reading from several meters away.
What marking materials do you recommend?
We offer hybrid barcode/QR labels + RFID chips suitable for critical environments. They facilitate fast scanning, traceability, and resistance to the stresses of the pharmaceutical industry.
Can I generate customized reports?
Absolutely. Our tools allow you to configure your own dashboards, schedule automatic exports, and generate customized reports based on your metrics.
Can I connect Solid to my LIMS or MES?
Yes. Our solutions are compatible with leading LIMS and MES systems thanks to our RESTful APIs, webhooks, and standard connectors.
How much of a reduction in tasks can I expect?
Our customers see a reduction of 1.5 to 1.8 FTE per 10,000 samples per year, with a return on investment of between 12 and 14 months. For a detailed example, see our article: How to automate sample tracking?
Are your RFID tags resistant to chemicals?
Yes. We offer labels specifically designed to withstand solvents, high/low temperatures, and corrosive environments.
How do our Solid solutions enable drug traceability?
Thanks to RFID technology and the digitization of your flows, our solutions ensure accurate tracking of your medicines at every stage of the production and distribution chain.
Any other questions?
Other sectors

Let's discuss your traceability challenges
Our experts are on hand to help you identify the solution best suited to your business challenges.
Join the demanding companies who trust Solid to track and optimize their assets and products on a daily basis.



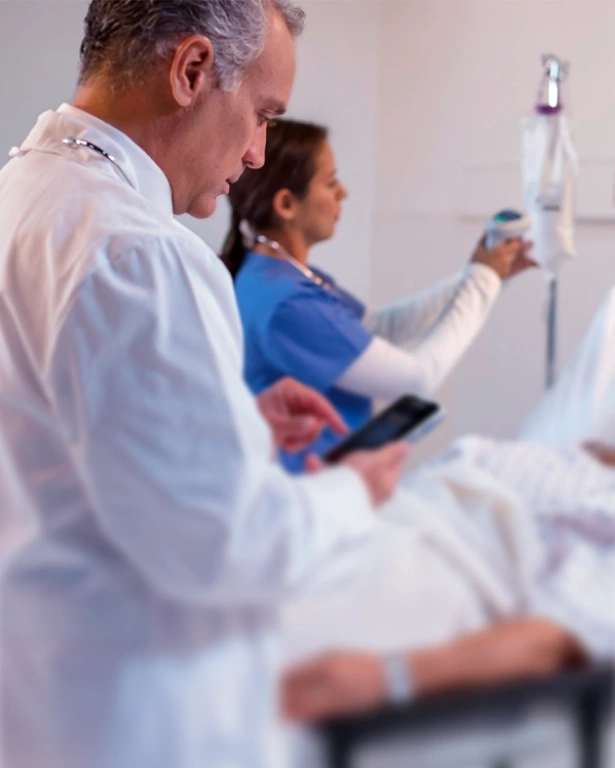

Our reports are automatically generated in accordance with FDA 21 CFR Part 11 and EU GMP Annex 11 standards. Each action is time-stamped, logged, and can be reinforced with a blockchain signature to guarantee data integrity and compliance.